The word “Jacobio” is a combination of the Hebrew name “Jacob” that can be translated as “to seize”, and “bio” from the root word of biomedical. The combination refers to our overarching goal to seize development opportunities in the life sciences and biomedical industries.
In our logo, the letter “i” is represented by the Rod of Asclepius, alluding to medicinal science. It has a clear meaning: We consider it our responsibility to develop novel and improved medicines to cure diseases, serve the public and mirror the World Health Organization’s vision of helping “all people attain the highest possible level of health.”
The life sciences sector has evolved rapidly over the past century. We aim to seize new opportunities in drug R&D that are created by breakthroughs in basic research and bring more therapies to patients.

We develop novel drugs with a professional attitude and embrace the challenges of diseases with elegance.
The history of anti-cancer drug R&D is relatively recent, with the first cancer patient on a clinical trial enrolling 80 years ago.
The cancer biology has evolved rapidly over the past half century. We aim to seize new opportunities in drug R&D that are created by breakthroughs in basic research and bring more efficient therapeutic options to cancer patients.
Click Company Culture to learn more.
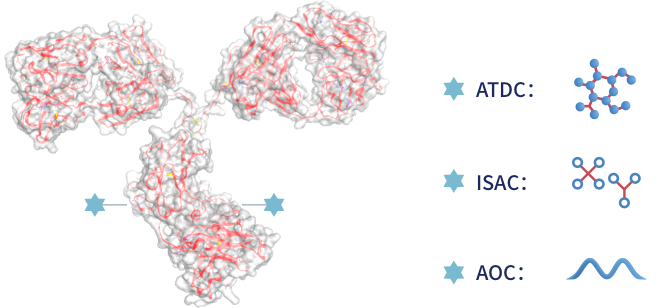
Small molecule drugs and monoclonal antibodies are widely used in cancer treatments and conventional antibody-drug conjugates (ADCs) with toxins as payloads are emerging. A growing body of clinical evidence shows that conjugated drugs such as ADCs can generate significant benefits for patients.
Targeting six classic oncogenic signaling pathways, Jacobio is developing the next generation of antibody-drug conjugates, including ATDCs (antibody-targeted drug conjugates), ISACs (immune-stimulating antibody conjugates), and AOCs (antibody oligonucleotide conjugates).
We use targeted drugs, immune-stimulators, or oligonucleotides as payloads, rather than using toxins as payloads, to further improve efficacy and safety.