Jacobio independently developed the SHP2 inhibitors, Sitneprotafib(JAB-3312) , using a drug design platform exploiting the allosteric binding site. Jacobio is currently conducting clinical trials in China and the United States. Both JAB-3312 have been granted orphan drug designation for esophageal cancer (including esophageal squamous cell carcinoma) by the U.S. FDA. Jacobio’s SHP2 inhibitor is the second to enter clinical development in the United States.
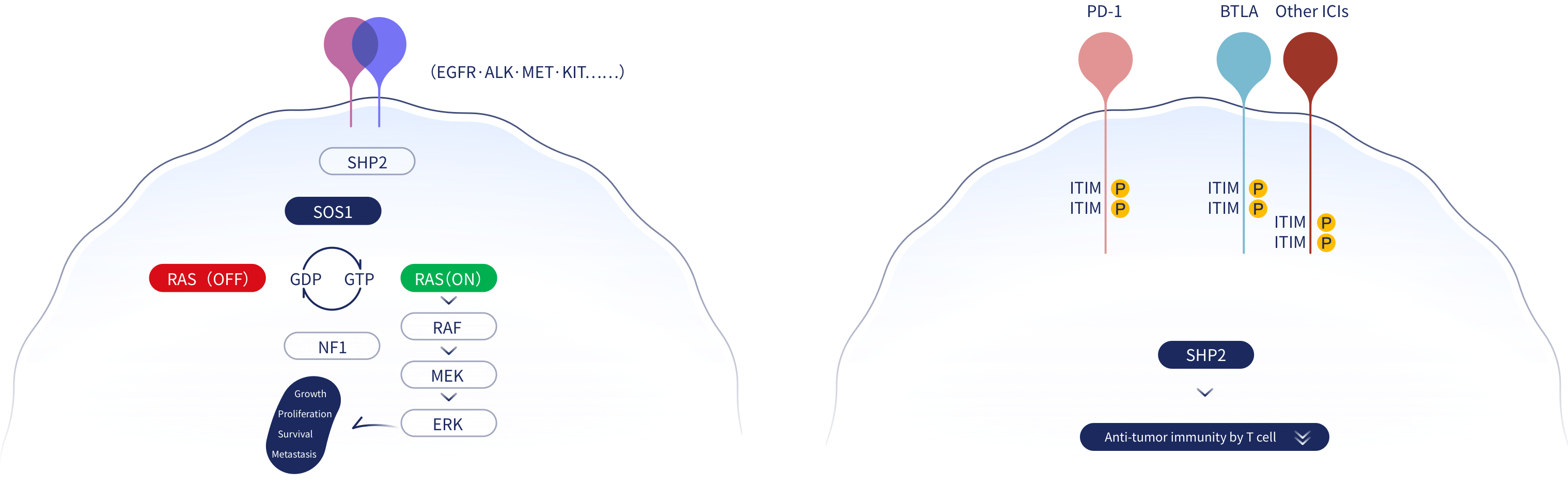
SHP2, also known as PTPN11, is a phosphatase that dephosphorylates substrate proteins. It is an important molecule regulating many cellular functions. In the receptor tyrosine kinase (RTK) pathway, SHP2 effects are upstream of RAS, in mediating tumor proliferation and downstream of the immune checkpoint regulator, Programmed cell death protein 1 (PD-1), in inhibiting the anti-tumor effect of T cells. SHP2 also functions downstream of Colony Stimulating Factor 1 Receptor (CSF1R), in promoting tumor-associated macrophage activity. Thus, targeting SHP2 has potential for multiple anti-tumor effects.
JAB-3312 is highly selective, allosteric inhibitors of SHP2 that can block the RTK/RAS/MAPK signaling pathways and inhibit the growth and proliferation of tumor cells driven by RTK or with KRAS, BRAF Class 3 and NF1 loss of function (LOF) mutations. In addition, JAB-3312 can block PD-1 signaling, enhancing the tumor-killing functions of CD8+ T cells, and relieve immunosuppression in the tumor microenvironment from tumor-associated macrophages resulting in overall anti-tumor effects.
Results from studies to date suggest that JAB-3312 may be effective in patients with non-small cell lung cancer, head and neck squamous cell carcinoma, esophageal squamous cell carcinoma, colorectal cancer, pancreatic cancer harboring specific gene mutations, and patients with Class 3 BRAF-mutated, or NF1 LOF mutated solid tumors. Estimates from the global tumor incidence data from 2019 indicate that 1.2 million patients with advanced solid tumors worldwide may benefit from SHP2 inhibitor monotherapy.
Also promising are synergistic effects for SHP2 inhibitors used in combination with a variety of targeted therapies and immunotherapies, such as KRAS/EGFR/ALK inhibitors, and PD-1 antibody, that would expand this potential benefit to even more patients.
|
Assets |
Partners |
Region |
Phase |
Indications |
Registration Information |
China Partner |
Jacobio Rights |
|
|---|---|---|---|---|---|---|---|---|
|
JAB-3312 |
Glecirasib (KRAS G12Ci) |
China | IIa | Advanced Solid Tumor |
ClinicalTrials: NCT05288205 CDE Number: CTR20220587 |
 |
Global rights |
|
| JAB-3312+Glecirasib VS Tirelizumab+Pemetrexed+Carboplatin | China |
III |
NSCLC |
ClinicalTrials: NCT06416410 CDE Number: CTR20241931 |
||||

Jacobio Pharma presented the stratified analysis data of PD-L1 expression for the combination of glecirasib and JAB-3312 in a poster at the 2024 European Society for Medical Oncology (ESMO) Annual Meeting