- 中
- 繁
- EN
Beijing, Shanghai, Boston, Apr. 17, 2024— Jacobio Pharma (1167.HK) announced that its in-house developed KRAS G12C inhibitor glecirasib has received orphan drug designation for pancreatic cancer indication from FDA. Previously, glecirasib had been granted Breakthrough Therapy Designation by CDE (Center for Drug Evaluation, NMPA) for the treatment of second-line or above pancreatic cancer patients with KRAS G12C mutations.
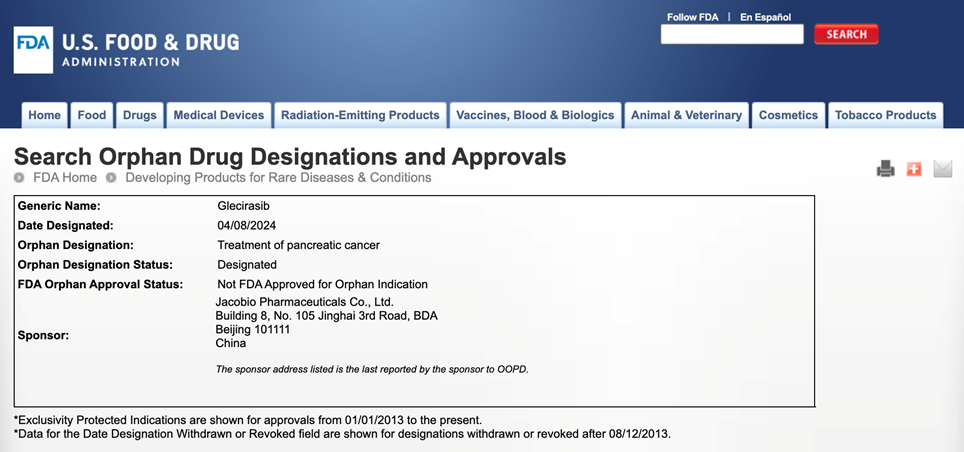
Pancreatic cancer is a highly malignant tumor, and current patients lack effective standardized treatment methods, with a five-year overall survival rate of only 3.1% in distant stage population. The orphan drug designation will help expedite the review and registration of glecirasib and expedite early access to the drug for patients.
Previously, Jacobio has announced data of glecirasib in patients with pancreatic cancer in the oral abstract session at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium (2024 ASCO GI). For second-line and above KRAS G12C mutated pancreatic cancer patients, the cORR was 41.9% (13/31) and the DCR was 93.5% (29/31). The median progression-free survival (mPFS) was 5.6 months, and the median overall survival (mOS) was 10.7 months.
Jacobio continues to explore the application of glecirasib in pancreatic cancer. Glecirasib’s registrational pivotal study for pancreatic cancer was approved by CDE in July 2023, which became the first global pancreatic cancer KRAS G12C registrational clinical study. The study results will be used to submit NDA (New Drug Application) for pancreatic cancer.
About Glecirasib
Glecirasib is a KRAS G12C inhibitor developed by Jacobio. A number of Phase I/II clinical trials of glecirasib are currently ongoing in China, the United States and Europe for patients with advanced solid tumors harboring KRAS G12C mutation. These include a pivotal clinical trial in NSCLC in China; a monotherapy study for STK11 co-mutated NSCLC in the front-line setting, and combination therapy trials with SHP2 inhibitor JAB-3312 in NSCLC and with Cetuximab in colorectal cancer. The pancreatic cancer indication has obtained orphan drug designation in the United States and breakthrough therapy designation in China.
Jacobio Pharma (1167.HK) is committed to developing and providing new and innovative products and solutions to improve people's health. Our pipeline revolves around novel molecular targets on six major signalling pathways: KRAS, immune checkpoints, tumor metabolism, P53, RB and MYC. We aim for our key projects to be among the top three in the world. Our vision is to become a global leader recognized for our impact in drug R&D together with our partners. Jacobio has R&D centers in Beijing, Shanghai and Boston with our Induced Allosteric Drug Discovery Platform (IADDP) and our iADC Platform.